Suspect XLH?
Connect their symptoms and diagnose with the applicable tests.
XLH is hereditary, chronic, and progressive1
X-linked hypophosphatemia (XLH) is a lifelong, progressive disease characterized by hypophosphatemia due to increased fibroblast growth factor 23 (FGF23) activity.1 It is considered rare—XLH affects up to 1 in 20,000 people in the US. Although XLH is primarily an inherited disease, 20% to 30% of cases arise spontaneously.1
XLH is the most common cause of inherited phosphorus wasting and leads to poor bone mineralization, resulting in rickets and osteomalacia.1,2
You and your patients may also refer to XLH by different names:3,4
XLH impacts the skeletal, muscular, and dental health of children and adults throughout their lives.1

What causes XLH?
Increased FGF23 activity is the underlying cause of chronic hypophosphatemia1
In normal homeostasis1
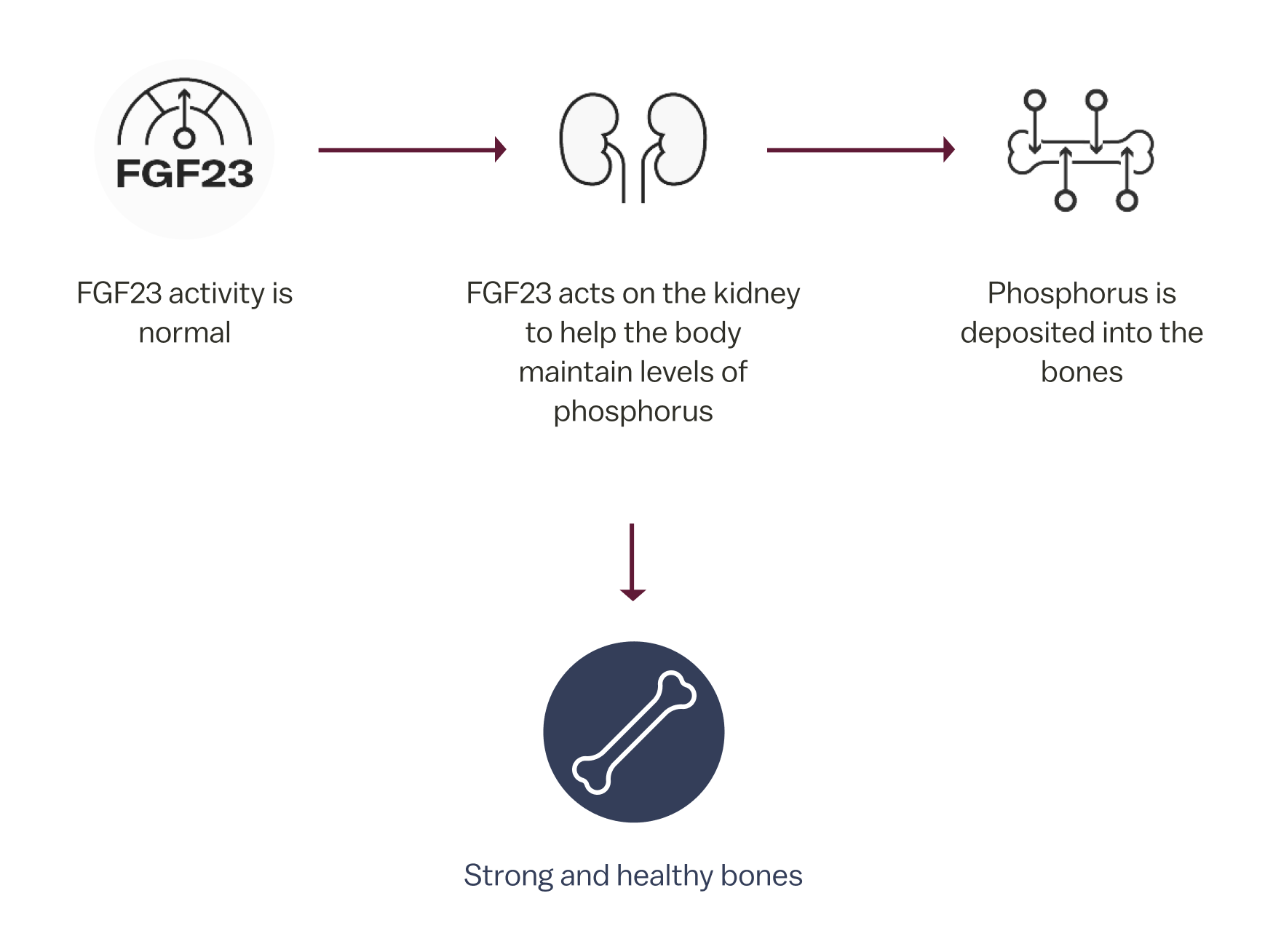
In normal homeostasis, FGF23 is a protein hormone produced by osteocytes in the bones that regulates serum phosphorus levels.1
In XLH1
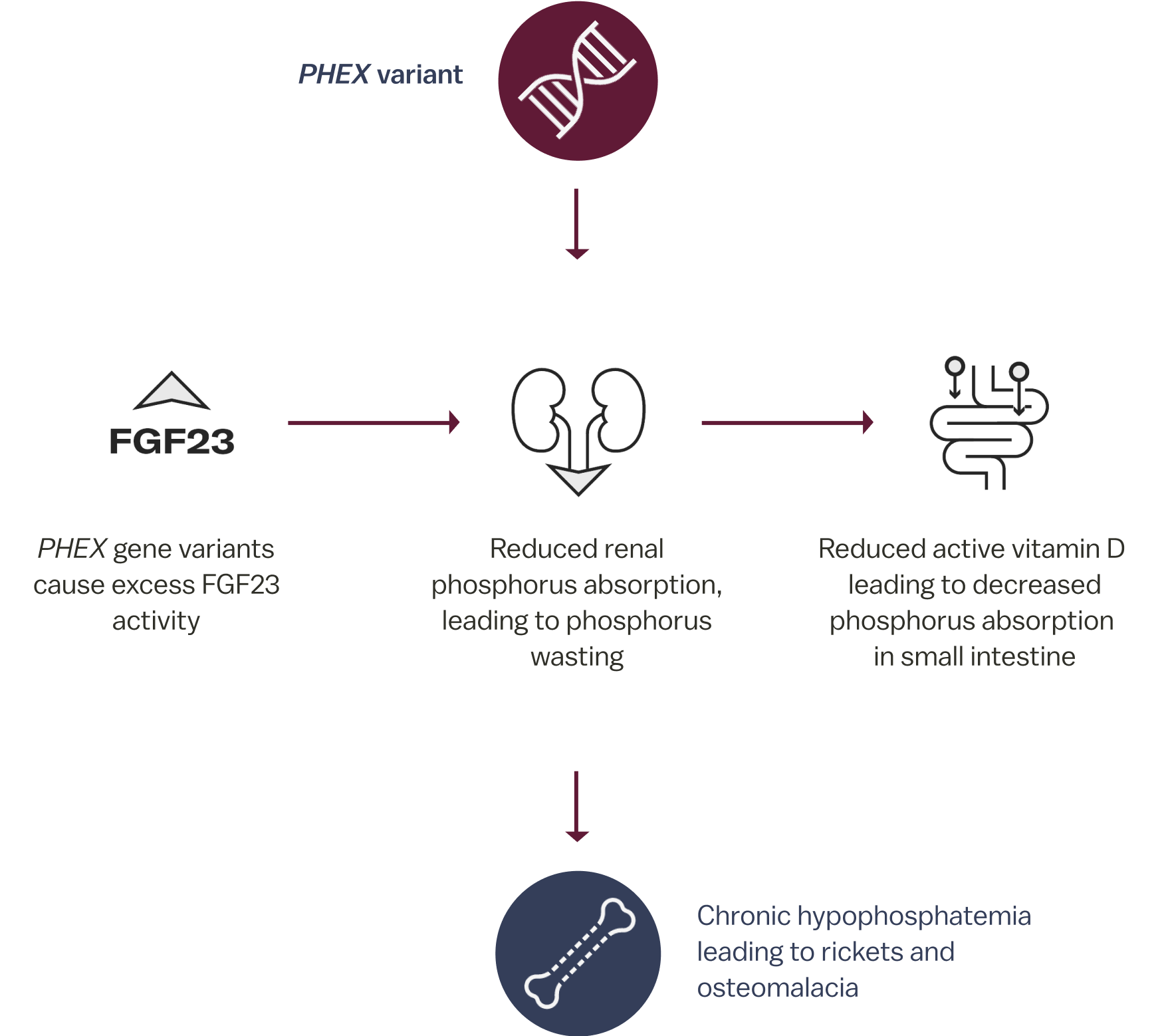
In XLH, a variant in the PHEX gene causes excess FGF23, which results in phosphorus wasting leading to chronic hypophosphatemia. Due to increased FGF23 activity, patients with XLH may experience skeletal defects, muscular dysfunction, and dental abnormalities.1,5

XLH affects the entire body. Chronically low levels of phosphorus in XLH impact bone formation, dental health, muscle functioning, and energy levels. The progressive nature of the disease can leave patients susceptible to delayed growth, fractures, limited physical functioning, and pain.5,8
These issues can impact the social and emotional wellness of children and adults.5 Getting an accurate diagnosis early can help minimize the burden of XLH.

Find resources for you and your patients to understand more about this rare condition.
References:
1. Dahir K, et al. J Endocr Soc. 2020;4(12):bvaa151. doi:10.1210/jendso/bvaa15 2. Haffner D, et al. Nat Rev Nephrol. 2019;15(7):435-455. 3. Ruppe MD. X-Linked Hypophosphataemia. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. 4. Fuente R, et al. Int J Mol Sci. 2022;23(2):934. 5. Hamilton AA, et al. J Endocr Soc. 2022;6(8):bvac086. 6. Carpenter TO, et al. J Bone Miner Res. 2011;26(7):1381-1388. 7. Trombetti A, et al. Nat Rev Endocrino. 2022;18(6):366-384. 8. Skrinar A, et al. J Endocr Soc. 2019;3(7):1321-1334.